Crustal Formation
If we take a chuck of crust and measure what it’s made of, we see that the crust is primarily composed of oxygen, silicon, aluminum, iron, sodium, potassium, magnesium, and calcium, with a smattering of other minor and trace elements (Rudnick & Gao, 2003). This composition is similar to that of the mantle and Bulk Silicate Earth, but differs quite a bit from that of the Earth as a whole (due mainly to iron accretion in the core). From this, we may conjecture that the crust was formed from the mantle some time after the mantle and core had separated, and the slight differences in composition arises from elemental behavior during the formation process.
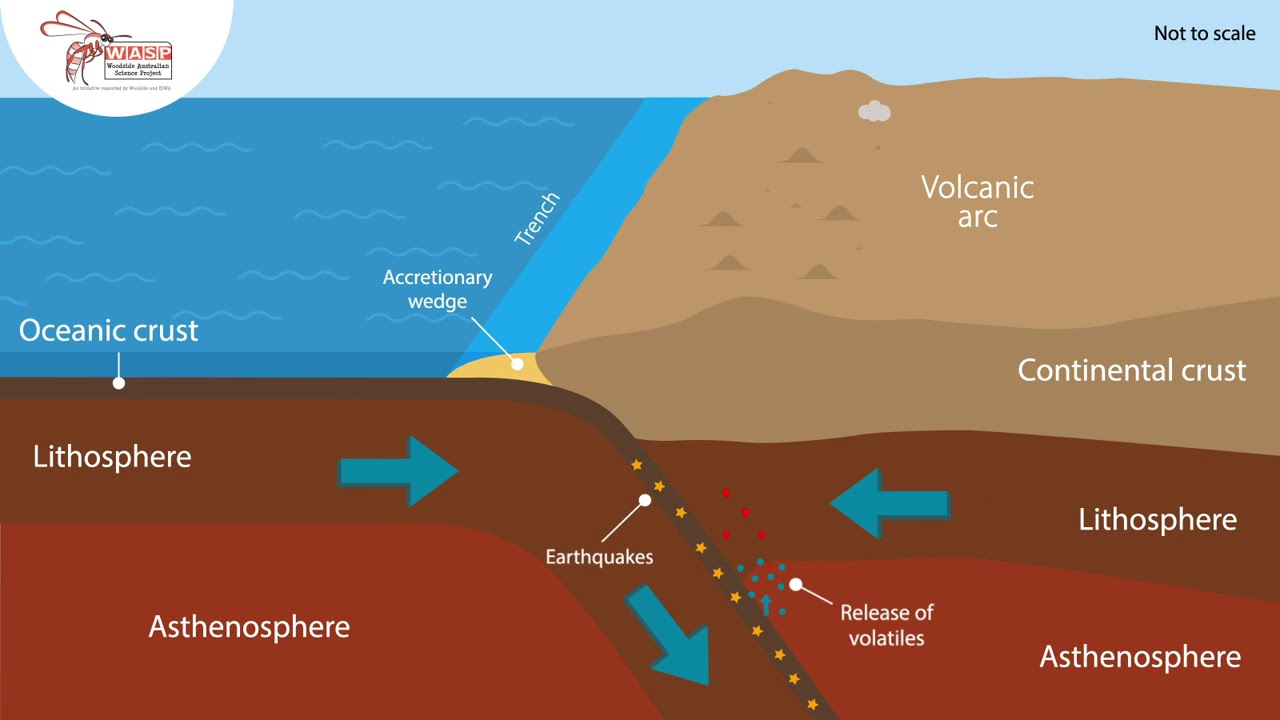
Geoscientists now believe that the crust was formed by partial melting of the mantle. Specifically, oceanic (basaltic) crust is formed mainly by decompressional melting of mantle rock at mid-ocean ridges (Mutter, Buck, & Zehnder, 1988). Hence, oceanic crust is quite homogeneous in nature and relatively uninteresting. Continental crust, however, is formed by the repeated melting of oceanic crust, and the more steps involved, the more heterogenous the result. As a result, continental crust is much more varied in composition, chemical, and physical properties when compared to oceanic crust.
The process of crustal formation through partial melting explains the differences in crust and mantle composition. In short, every element has a partition coefficient (D) describing whether it prefers the solid mantle rock or the melt that will eventually become crustal rock. If an element is compatible with the solid phase (D > 1), it will become depleted in the crust, but if an element is incompatible with the solid phase (D < 1), its concentration will be enhanced in crustal formations. If additional melting occurs, perhaps to form continental crust, the incompatible elements will become increasingly enhanced in the melt, such that their concentration in the continental crust may be noticeably higher than in oceanic crust.
We know that the crust wasn’t formed all at once. New oceanic crust is being formed continuously at divergent plate boundaries, and the repeated melting to form continental crust is also an ongoing process. Naturally, given some crustal location, we want to know how old the crust is. To do this, we can turn to the presence of rare but important isotopes in the crust: those that are radioactive.
Rubidium-Strontium Systems
Soon after the discovery of radioisotopes, geochemists realized that we could use the decay of radioactive isotopes with a known half-life to date objects and events from a distant past. Some may be familiar with radiocarbon (Carbon 14) dating, which is the preferred dating method of archaeologists, but the 5730 year half-life of C-14 is too short to be of much use to many geochemists interested in Earth’s history. Instead, in the late 1930s, scientists formalized the so-called “Strontium Method”, using the beta decay of rubidium-87 into strontium-87 with a half-life of around 49 billion years (Faure & Powell, 1972). 49 billion years is, of course, much greater than the age of the Earth, and so this system can be used to date objects from Earth’s deep past.
While the decay of Rb-87 into Sr-87 has a long half-life, it is not unique among decay systems in that regard. Instead, the usefulness of rubidium and strontium stems in large part from their geochemical behavior during partial melting processes. While strontium and rubidium are both trace elements, they are also very incompatible with the solid mantle phase (D << 1), and so are enriched in crustal rocks formed from mantle melt. In particular, rubidium is more incompatible than strontium, so in many crustal rocks we can see a measurable amount of rubidium (Shimizu, 1972). There is also no ambiguity in this system; Rb-87 is the only naturally occurring radioactive isotope of rubidium, and Sr-87 is the only naturally occurring radiogenic isotope of strontium. Finally, different minerals in any given rock all have different rubidium concentrations, a requirement for many advanced dating techniques.
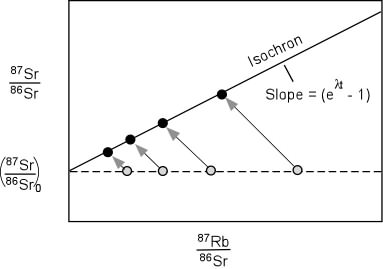
Specifically, rubidium in the continental crust lends itself perfectly to the use of the isochron method of radiometric dating (pictured below). The isochron method tracks the radioactive decay in individual members of a system as they move concurrently through time. If a system includes a variety of different rocks and minerals, each formed at the same time but containing different amounts of the radioisotope and with a uniform Sr-87/Sr-86 ratio, then at any time, the ratio of Sr-87/Sr-86 to Rb-87/Sr-86 should fall on a line whose slope gives the age of the sample. Because rubidium fractionates preferentially into the continental crust but in varying amounts in different minerals, the isochron dating method is perfect for rubidium-strontium dating.
Special Applications
To understand the power of the Rb-Sr dating system, let us take a short trip to Shetland, Scotland. Sampling a variety of rocks and applying the Rb-Sr dating technique, scientists could determine the ages of many minerals in the rocks, including the micas biotite and white mica (mostly muscovite), most of which were formed between 400 and 500 million years ago (Walker, Thirlwall, Strachan, & Bird, 2016). Being able to date rocks from so long ago is already impressive, but the precision with which Rb-Sr dating allows us to do so is even more extraordinary. Most results carried an uncertainty so small that the error bars fit within the marker symbol, indicating an uncertainty of at most a few million years for a date of a few hundred million years.
While the reasoning process is a bit more involved, the results of Rb-Sr dating the Shetland rocks also allowed researchers to conjecture about metamorphic processes, specifically when and under what conditions they may have occurred. For instance, the difference in white mica and biotite ages indicates that a later event altered the composition of biotite in some rocks, and the white mica ages of heavily deformed rock samples indicate that a late Ordovician orogenic (mountain-building) event may have extended further than previously believed (Walker, Thirlwall, Strachan, & Bird, 2016). More generally, these metamorphic processes often correspond to larger-scale regional tectonic processes, and understanding these tectonic processes can aid us in our goal of reconstructing the tectonic history of Earth’s continents.
References
Faure, G., & Powell, J. L. (1972). Measurement of Geologic Time by the Rubidium-Strontium Method. Strontium Isotope Geology, 9–22. https://doi.org/10.1007/978-3-642-65367-4_2
Mutter, J. C., Buck, W. R., & Zehnder, C. M. (1988). Convective partial melting: 1. A model for the formation of thick basaltic sequences during the initiation of spreading. Journal of Geophysical Research, 93(B2), 1031. https://doi.org/10.1029/jb093ib02p01031
Rudnick, R. L., & Gao, S. (2003). Composition of the Continental Crust. Treatise on Geochemistry, 3, 1–64. https://doi.org/10.1016/b0-08-043751-6/03016-4
Shimizu, N. (1974). An experimental study of the partitioning of K, Rb, Cs, Sr and Ba between clinopyroxene and liquid at high pressures. Geochimica et Cosmochimica Acta, 38(12), 1789–1798. https://doi.org/10.1016/0016-7037(74)90162-8
Walker, S., Thirlwall, M. F., Strachan, R., & Bird, A. (2016). Evidence from Rb–Sr mineral ages for multiple orogenic events in the Caledonides of Shetland, Scotland. 173(3), 489–503. https://doi.org/10.1144/jgs2015-034
For educational purposes only: UCLA EPSS 113C